Automated analysis of copper concentrates
The need for analysing copper concentrates to determine the copper content accurately and rapidly is increasing.
Hence, there exists a demand for automated systems that perform the wet chemistry methods for the analysis of copper concentration in powder samples. Automation brings the advantage of increased hands-off capacity and improving accuracy and precision for routine analysis. It may even come with a considerable reduction in process cost.
Automation also provides an effective solution to the tedious process of sample preparation that expose laboratory staff to major safety risks when working with concentrated aggressive chemicals like boiling acids, strong bases and exposure to hydrofluoric acid vapours.
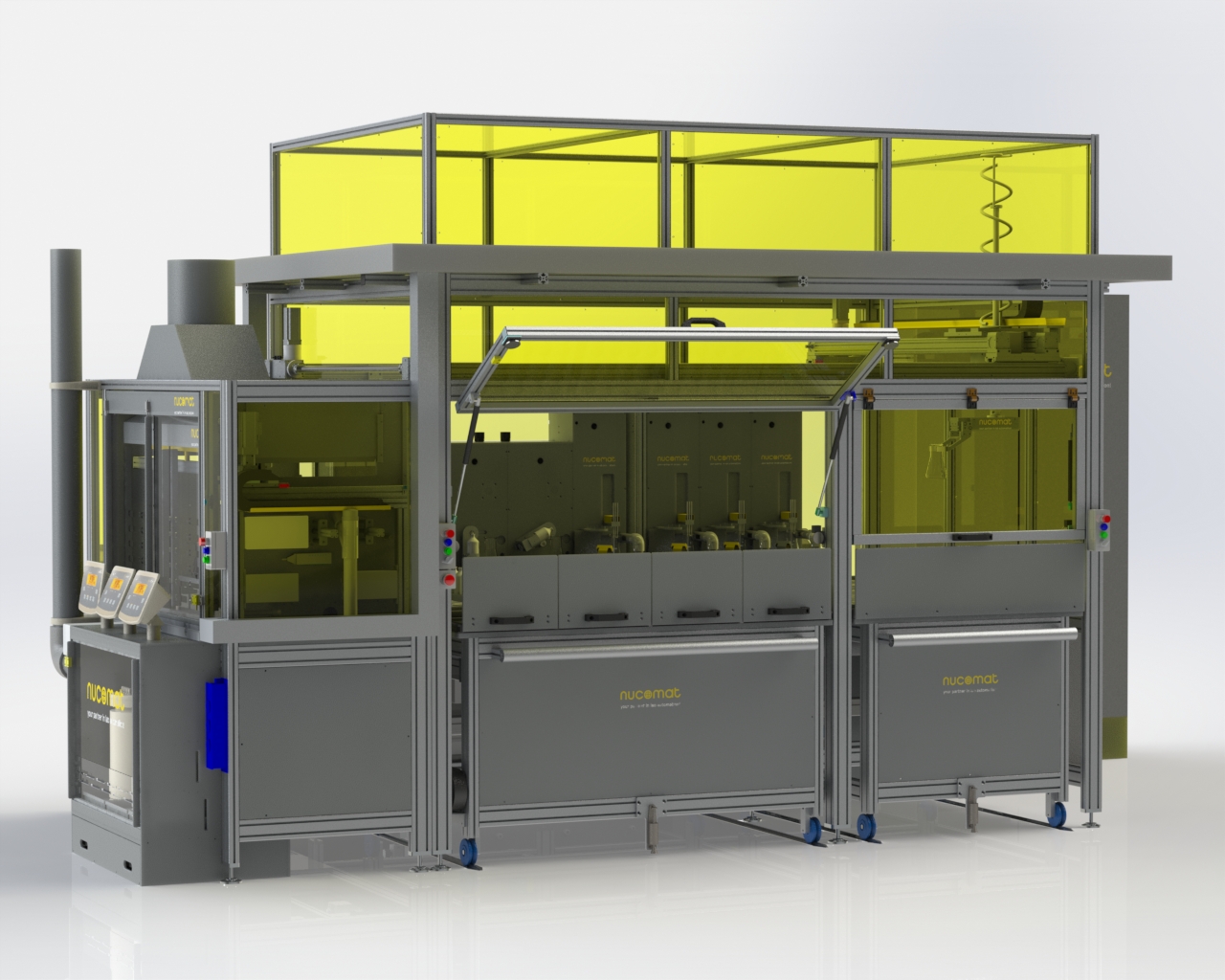
Nucomat has engineered an automated platform for determining the typically between 10% to 50% copper in concentrated samples. The platform provides industrial scale analytical capacity to process samples. Starting with gravimetrically dispensing replicate powder samples at pre-set weights (between 100 and 1000 mg), followed by automated Hotcell acid dissolution and leaching steps and finally performs Iodometric titration using the colour change of starch as readout, similar to the manual process. Calibration of the titrant is performed with pure copper reference material. Click here for the video
Materials and methods
Sample dissolution is performed with hot acids. For samples containing between 20% and 30% copper about 400mg of the dry powder are dispensed in replicates into titration flasks and the sample weight recorded. A mixture of HNO3 (68%), HCl (32%) and HClO4 (70%) is dispensed in 10/5/3 volume ratio into the titration flask and heated to leach for up to 12 minutes on the Hotcell.
Pure copper is used as reference sample. 100 mg of reference sample is dispensed and dissolved in HNO3 (68%) on the Hotcell, without addition of HCl or HClO4. Excess liquid is evaporated from all samples for up to 20 minutes until an almost dry syrupy state is left at the bottom of the flask. After a cooling period the salts are dissolved in 25 mL water, re-heated for 5 minutes and allowed to cool down again on a swirl station.
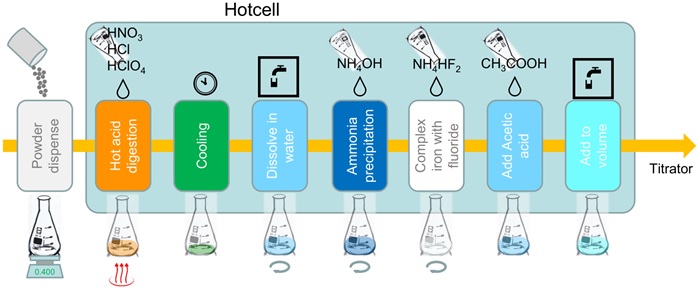
To neutralise the excess of acid 5 mL ammonia (25%) is added. Both iron and copper hydroxide are precipitated. The iron that will interfere with the titration is complexed with 20 mL NH4HF2 as a 20% (w/v) solution that is prepared fresh daily. Water is added to dilute the sample to about 100 mL and the sample acidified by adding 5 mL of glacial acetic acid.
The iodometric titration is based on oxidation of iodides to iodine by copper (II) ions, which get reduced to Cu+ upon addition of excess KI. (2Cu2+ + 4I– → 2CuI(s) + I2). 30 mL of a 10% (w/v) KI solution (made fresh daily) is added to the titration flask while mixing. The solution turns into a yellow-brown suspension. Thiosulfate titrant addition will convert free I2 back to I–, diminishing the blue colour from the starch – I2 indicator complex. (2S2O32- + I2 → S4O62- + 2I–). Titrant solution is made fresh daily with 19.618 g/L Na2S2O3.5H2O and 25 mg/L NaOH. The titration is performed in 3 subsequent time series under video monitoring. First titrant is added until discolouration of the KI suspension. To the white suspension 2 mL of a 1% fresh starch solution is added. Secondly the titration is continued until the violet-blue colour is almost gone. Just before the equivalence point 5 mL of NH4SCN 10% (w/v) is added to release the last traces of I2 present, turning the solution again intense violet blue. Finally, titrant is added slowly until the equivalence point is reached with transition to a white solution as detected by the video titrator. The process is optimised in real-time by monitoring the colour changes and adjusting titration speed throughout the titration process: Fast in approach, slow and accurate near equivalence.
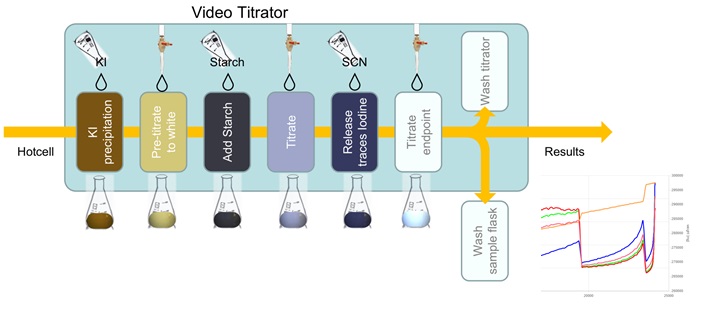
All data from standards, sample replicates, individual reagent additions, and endpoint calculations are logged in the database with the sample replicate ID. Automated cleaning routines enable re-use of titration flasks on the system and prepare the titrator for running new samples without carry-over, and fully hands-off.
The standard automation setup with a capacity of up to 250 titrations daily, comprises:
- 2 powder samplers: Automatically dispense and accurately weigh samples into titration flasks with gravimetric control.
- 16 position Hotcell: A robotized fume hood for safe acid sample dissolution and leaching at high temperature.
- 2 acid and reagent dispensers: prepare the reaction steps inside the Hotcell, accurately adding liquids like acids, bases and reagents with gravimetric control.
- 1 swirling position: mix samples following addition steps and cool samples in the Hotcell.
- 2 wash stations to automatically clean, rinse and re-use titration flasks after analysis.
- 4 video imaging titrators: Proprietary titration stations to run colour titrations were specifically developed to replace manual titration. LED illumination ensures constant imaging conditions. The compact industrial inspection cameras reproducibly record the titration process for live video analysis of the region of interest (ROI) within the liquid area on the field of view. The titrator stations are equipped with mixer and reagent addition. Subsequent additions of titration reagents are gravimetrically reported and can be monitored by video analysis. A high precision syringe for titrant addition is operated dynamically under video analysis to perform fast and precise end-point titration. Automated station cleaning cycles reduce maintenance and prevent sample carry-over.
Space saving overhead mounted Cartesian robotics move sample flasks from one workstation to the next according the protocol and scheduled by priority. Loaded samples are tracked throughout the process by their sample identification. Workstations are operated by microcontrollers to autonomously execute their task based on supplied sample parameters. Returned performance results after each task are logged in the sample database and linked to the sample identification. The gravimetric control on every single addition makes each individual step of an analytical method traceable and highly reproducible. Error trapping, smart retry, recovery with sample abort and automatic aliquot regeneration ensure every entered sample produces a validated result.
Variability:
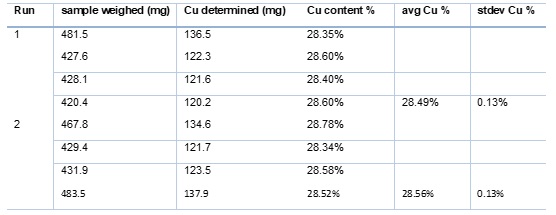
Running a variety of ore samples prepared as fine powder, we have demonstrated high precision readout with standard deviations as low as 0.13% on samples having near 28% copper content. When comparing 2 sets of quadruplicate runs on the same sample again the same standard deviation was obtained, and the average sample quantification between the 2 runs was only 0.07% apart.
Download the full white paper here:
Cu concentrate.pdf